vartmaan institute sirsa
Unit I: Electrostatics
Chapter–1: Electric Charges and Fields
Electric charges, Conservation of charge, Coulomb’s law-force between two point charges, forces between multiple charges; superposition principle and
continuous charge distribution.
Electric field, electric field due to a point charge, electric field lines, electric
dipole, electric field due to a dipole, torque on a dipole in uniform electric field.
Electric flux, statement of Gauss’s theorem and its applications to find field
due to infinitely long straight wire, uniformly charged infinite plane sheet and
uniformly charged thin spherical shell (field inside and outside).
To download notes click on the link given at just end of the topics
Chapter–1: Electric Charges and Fields
Chapter–2: Electrostatic Potential and Capacitance
Electric potential, potential difference, electric potential due to a point charge,
a dipole and system of charges; equipotential surfaces, electrical potential
energy of a system of two-point charges and of electric dipole in an
electrostatic field.
Conductors and insulators, free charges and bound charges inside a
conductor. Dielectrics and electric polarization, capacitors and capacitance,
combination of capacitors in series and in parallel, capacitance of a parallel
plate capacitor with and without dielectric medium between the plates, energy
stored in a capacitor. loss of energy in sharing of capacitor
To download notes click on the link given at just end of the topics
Chapter–2: Electrostatic Potential and Capacitance
Unit – I Electrostatics
- Electrostatics:-
The branch of physics which deals with the study of charges at rest & the forces & fields, potential of charges is called electrostatics.
* Electrostatics was discovered around 600Bc by a Greek philosopher Thales of Miletus. He showed that when amber was rubbed with a cloth, then cloths starts attracting small piece of paper. In Greek amber is called electron so phenomenon was called electricity. (Amber is a yellow gum like substance obtained from old plants).
* In 1544 – 1603 Sir William Gilbert found that a force was present after rubbing of amber or any other substance called electrostatic force.
* In 1731 Stephen gray found that charge can be moved through a metal for a long distance but not through a thread. This leads to two types of materials
(i) Conductor (ii) Insulator.
* In 1733 Du Fay discovered that charges are of two types + ve charge – ve charge.
* In 1750 Benjamin Franklin proposed one fluid theory. He believed that charge occurs due to transfer of electrons. The excess electrons means -vely charged body & deficiency of electrons means + vely charged body.
- What is electric charge:-
The property of electrons due to which they experience force of interactions is called electric charge.
The gravitational force of attraction between two electrons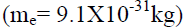 separated by 1 cm distance is
separated by 1 cm distance is
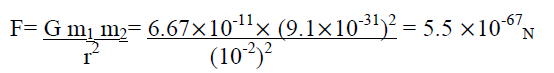
But it is found that two electrons separated by 1 cm distance repel each other with a force of 2.3 10-24N. This force is called electric force.
The charge on an electron is -1.6 10-19C & charge on an proton is+ 1.6 10-19C.
Neutrons have no charge though they have mass.
- Two kinds of charge:-
In 1733 Du Fay discovered that charges are of two types.

(i) Positive charge (ii) negative charge
This can be shown by the flowing simple experiments.
Exp-1. When two glass rods rubbed with silk are brought near each other then they started repel each other.
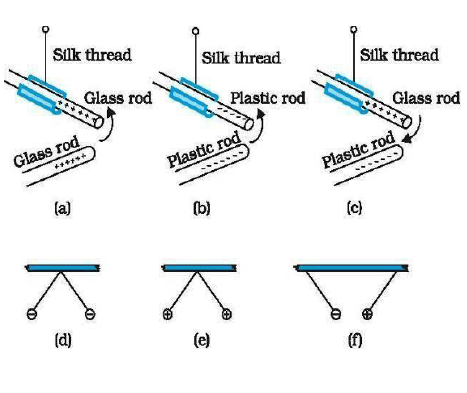
Exp.2 When two ebonite rods rubbed with cat’s fur are also broughtnear each other, then they also repeal each other.
Exp. 3:- But when a glass rod & ebonite rod are brought near each other then they started attracting each other.
Thus charges are of two types. Here we can see that like charges repel each other & unlike charges attract each other.
Here the property which differentiates the two kinds of charges is called polarity of charges.
* The charge on glass rod is called vitreous charge (Latin vitrum = glass) & the charge on amber when rubbed with wool is called resinous charge (Amber is resin)
Now according to Benjamin frankly, charges may define as.
- Positive charge:-The charge developed on glass rod when rubbed with silk is called + ve charge. Or if numbers of p are greater than number of e– in a body, then body is called +vely charged.
- Negative charge:-The charge developed on plastic rod when rubbed with wool is called negative charge. Or If number of e– is more than number of p+ in a body, and then body is called negative charged.
E, g:-Two kind of charge developed on rubbing
| Column – I (+ ve) | Column – II (- ve charge) |
| Glass rod, fur or cat skin, woolen cloth | Silk cloth ebonite rod Amber rod plastic, rubber |
4. Conductor, insulator & dielectrics:-
Conductor:- A substance which can be used to conduct electric charge from one place to another place is called conductor. Silver is the best conductor. Other examples are copper, aluminum, iron mercury, earth, human body etc.
Insulator:- A substance which cannot be used to conduct electric charge is called insulator
e, g :- glass, rubber, plastic, ebonite, mica, wax etc.
Dielectric:- Dielectric are those insulators which does not conduct electricity but on Appling external electric field charge induces on it e, g:- glass rod & paper acquire charge on rubbing.
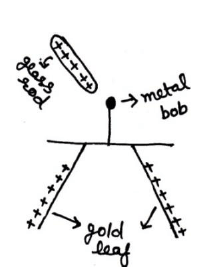
5. Gold leaf electroscope ( G L E )
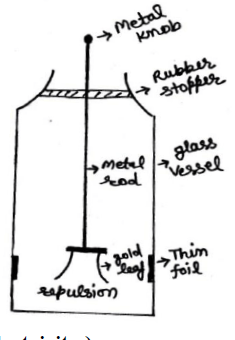
It is a device which is used for detecting an electric Charge &identifying its polarity. It is consist of a vertical conducting rod passing through a rubber stopper fitted in the mouth of glass vessel. Two thin gold leafs are attached to the lower end of the rod. When a charged object touches the metal knob at the outer end of the rod, the charge flow down throw the leaves. The leaves diverge (moves away) due to repulsion of the like charge. The degree of divergence of the leaves gives measure of the amount of charge.
6.Method of charge
(i) Charging by friction:-
When a body having loosely bounded electrons, is rubbed with a body having strongly bounded electrons, then both the bodies becomes charged by transfer of e–.
E,g:- when glass rod is rubbed with fur then glass rod become +vely charged by transferring electron & fur become -vely charged by acquiring electron.
- By loosing electron mass of the body decreases & by gaining e– mass of body increases. As electron have mass 9.1 10-32kg.
(ii) Charging by electrostatic induction:-
The phenomenon of charging a neutral body by placing it in the neighboring of a charge body is called electrostatic induction.
When a positively charge body is bring toward a neutral body then charge separation takes place in neutral body which remains till the + vely charged body remains near to the neutral body. These induced charges may be explained in following ways.
- Charging by induction ( by earthing a conductor);-
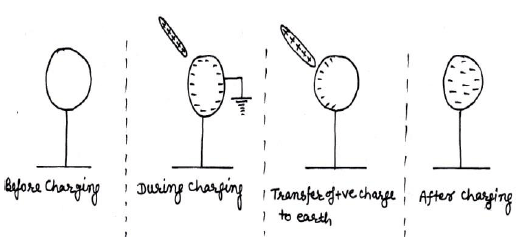
Suppose a neutral ball on an insulating stand & a positively charged glass rod is bringing toward it. Due to + ve charge on glass rod, the –ve char
- Charging by induction ( By separating conductors)

Q.1 how can you charge a metal sphere positively without touching it?
Ans: By bringing a negatively charged body towared metal sphere.
(iii) Charging by conduction (contact):-
Charging by conduction requires the actual contact between the two bodies.
In case of gold leaf electroscope when glass rod rubbed with silk is touched to the knob of leaf, than leaf diverse from their ac
tu
al position, which remains separated even after removal of glass rod. Thus charging may be done by conduction.
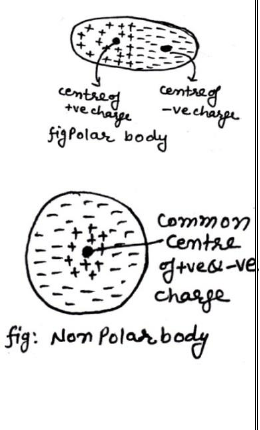
7. Polar & Non Polar Bodies:-
Polar body:- A body having different center of +ve& -ve charge is called polar body. E, g: – HCl , H2O etc.
Non polar body:-A body having same center of +ve& -ve charge is called non polar body. E, g: – H2, O2, N2, etc
Q.2. (a) A comb run through one’s dry hair attracts small bits of paper. Why? What happens if the hair is wet or if it is a rainy day? (Remember, a paper does not conduct electricity.)
(b) Ordinary rubber is an insulator. But special rubber tyres of aircraft are made slightly conducting. Why is this necessary?
(c) Vehicles carrying inflammable materials usually have metallic ropes touching the ground during motion. Why?
(d) A bird perches on a bare high power line, and nothing happens to the bird. A man standing on the ground touches the same line and gets a fatal shock. Why?
Solution (a) this is because the comb gets charged by friction. The molecules in the paper gets polarized by the charged comb, resulting in a net force of attraction. If the hair is wet, or if it is rainy day, friction between hair and the comb reduces. The comb does not get charged and thus it will not attract small bits of paper.
(b) To enable them to conduct charge (produced by friction) to the ground; as too much of static electricity accumulated may result in spark and result in fire.
(c) Reason similar to (b).
(d) Current passes only when there is difference in potential.
8. Some Basic Properties & electric charge:-
Same as mass, charge is also a fundamental & intrinsic property of matter. As like charges repel each other & unlike charge attract each other. There are three basic properties of charge.
(i) Quantization of charge (ii) conservative nature of charge (iii) Additive Nature of charge
- Quantization Of Charge:- (Discrete Nature of charge )
According to quantization nature of charge, the charge on a body is always whole number multiple of charge on an electron.
I,e charge on a body q = ne n= 1, 2, 3, 4, ……… n≠ ½, 3/2, 1.5 etc.
Where e = 1.6 10–19C (charge on an electron) & n is a whole number
- During rubbing, electron can transfer from one body to another body in a whole number. So charge is quantized as half electron cannot be transferred.
- A elementary particle quart of very small life time have fraction of charge.
± e & ± e. (it is exception of quantization of charge)
Q.3. If 109 electrons move out of a body to another body every second, how much time is required to get a total charge of 1 C on the other body?
Solution: Therefore the charge given out in one second is= 1.6 × 10–19 × 109 C = 1.6 × 10–10 C.
The time required to accumulate a charge of 1 C is
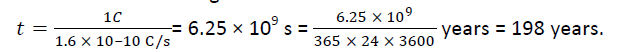
Thus to collect a charge of one coulomb, from a body from which 109 electrons move out every second, we will need approximately 200 years.
- One coulomb is, therefore, a very large unit for many practical purposes.
Q.4. how much positive and negative charge is there in a cup of water?
Solution: Let us assume that the mass of one cup of water is 250 g.
The molecular mass of water is 18g. so we can say 18g of water contain 6.022×1023 molecules
1g of water contain![]() molecules
molecules
Therefore the number of molecules in one cup of water is 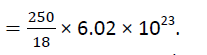
Each molecule of water contains two hydrogen atoms and one oxygen atom, i.e., 10 electrons and 10 protons.
Hence the total positive or total negative charge =
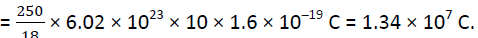
Q.5. A polythene piece rubbed with wool is found to have a negative charge of 3 × 10−7 C. (a) Estimate the number of electrons transferred (from which to which?) (b) Is there a transfer of mass from wool to polythene?
- When polythene is rubbed against wool, electrons get transferred from wool to polythene. Hence, wool becomes positively charged and polythene becomes negatively charged. Amount of charge on the polythene piece, q = −3 × 10−7 C
Amount of charge on an electron, e = −1.6 × 10−19 C
Number of electrons transferred from wool to polythene = n
as q = ne Therefore, the number of electrons transferred from wool to polythene is 1.87 × 1012 .
(b) Yes. There is a transfer of mass taking place. This is because an electron has mass, me = 9.1 × 10−3 kg Total mass transferred to polythene from wool, m = me × n = 9.1 × 10−31 × 1.85 × 1012 = 1.706 × 10−18 kg Hence, a negligible amount of mass is transferred from wool to polythene.
- Conservative nature of electric charge:-
According to low of conservation of electric charge, the total charge on an isolated system remains constant, it can neither be created nor be destroyed& can only be transferred from one body to another body.E,g:- (i)

Here charge on reactant is zero & charge on product = +1-1=0
So law of conservation of charge hold good.,
(iii) Addition nature of electric charge:-
According to additive nature of charge, the net charge on an isolated system can be simply obtained by adding all the charges scalarly.
If a system having charge q1, q2, q3 …qn then total charge on the system q = q1+ q2+ q3 ………… qn
E,g If system have four charges 2µC, 3µC, 4µC, -5µC
Then total charge q = 2µC+ 3 µC+ 4 µC+( -5 µC)= 4 µC
9. comparison of charge & mass :-
| Electric charge | Mass | |
| 1 | Electric charge may be + ve, -ve or zero. | The mass of a body is always a positive. |
| 2 | Electric charge is always quantized. | Quantization of mass is yet not obeyed. |
| 3 | Charge on a body does not depend on its speed. | Mass of the body increase with its velocity. |
| 4 | Charge is strictly conserved. | Mass is not conserved as it may convert into energy. |
| 5 | The electrostatic force between two charges may be attractive or repulsive. | Gravitational forces between two masses are always attractive. |
| 6 | Electrostatic force between different charges may cancel out. | Gravitational forces between different masses never cancel out. |
| 7 | A charged body always has some mass. | A body having mass may not have any charge. |
10. Coulombs law in electrostatics:- (Scalar form)
In 1785, the French physicist Charles Augustan coulomb measured the electric force between small charged spheres by using a torsion balance & gave a law which is as below.
Coulombs law states that the force of interaction between two stationary charge is directly proportion to the product of magnitude of charges & inversely proportional to the square of distance between the charges. This force acts along the line joining among the charges.
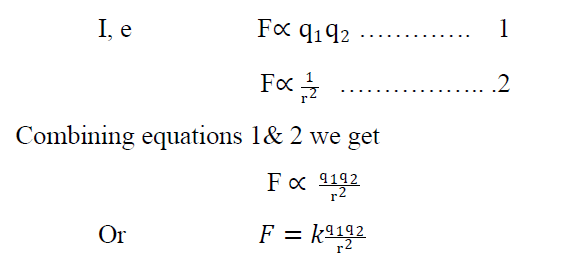
Where k is constant of proportionality & its value is
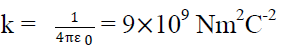
Where 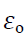 is a another constant called permittivity of free space & its value is
is a another constant called permittivity of free space & its value is
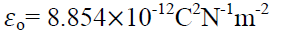
So coulomb’s law becomes
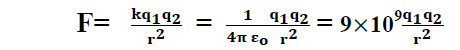
Unite of charge:-
The S.I unite of electric charge is 1 coulomb.
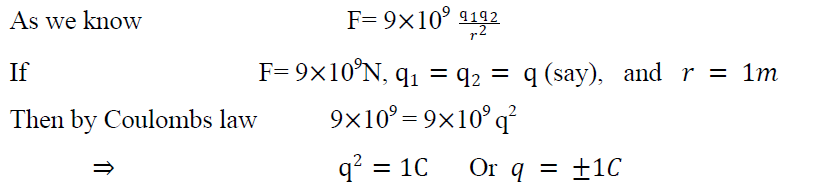
Hence one coulomb is that amount of charge which repels another equal charge with a force of 9 109N when placed one meter distance apart.
- The c, g, s unit of charge is stat Coulomb ( stat C ) or electrostatic unit of charge (emu )
One stat coulomb is that charge which repel an identical charge in vacuum at distance of 1 cm with a force of 1 dyne
1 Coulomb =3 109 statCoulomb (e m u)
- In electromagnetic c, g, s unit of charge is abCoulomb’s or electromagnetic unit of charge (e m u)
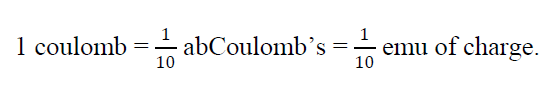
Significance of coulombs law:-
(i) It tells us about the force which bounds the electrons around the nucleus to form a atom.
(ii) It tells us about the force which binds the molecule to form solids & liquids
Limitations of coulomb’s law:-
(i) Coulombs law is applicable only on point charges.
(ii) It holds good only when charges are in rest only.
11. Comparison between coulomb’s force &Newton’s gravitational force:-
Similarity
(i) Both obey inverse square law.
(ii) Both forces are central forces.
(iii) Both forces are conservative forces.
(iv) Both forces are directly proportional to product of interacting Patrick
Dissimilarity:-
(i) Coulomb’s force is attractive as well as repulsive while Gravitational force is always attractive in nature.
(ii) Coulomb’s force is much stranger than gravitational force (1036times)
(iii) Coulombs force depends upon the medium in which charges are placed while gravitational force does not depends upon medium.
- Q. How much stronger is coulombs force from gravitational force between a electron & proton separated by r distance?
I,e
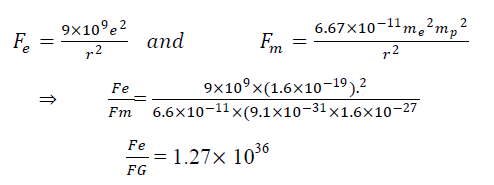
Hence Fe is 1036 time stronger then FG
- Q.6 : What is the force between two small charged spheres having charges of 2 × 10−7 C and 3 × 10−7 C placed 30 cm apart in air?
- Ans: 6×10-3N
- Q.7: The electrostatic force on a small sphere of charge 0.4 µC due to another small sphere of charge − 0.8 µC in air is 0.2 N. (a) what is the distance between the two spheres? (b) What is the force on the second sphere due to the first?
Ans: ![]()
(b) both have equal force so F=0.2N
12. Coulomb’s Law in vector from:-
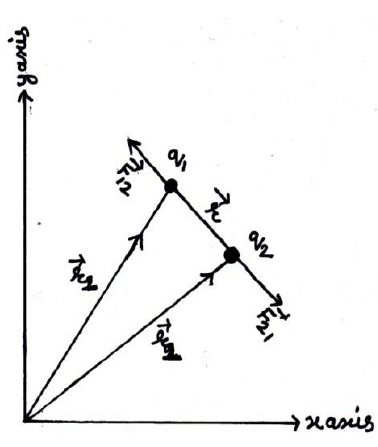
Suppose two charge q1& q2 placed at A & B having position vectors & from origion o. Now again suppose that charge q1 exert force on q2& q2 exerts force on q1. Then from coulomb’s Law
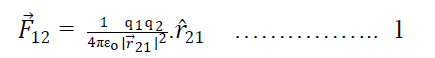
Where is a position vector acting from q2 to q1.
Again from Coulomb’s Law force on q2 to q1
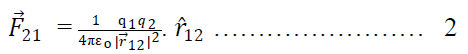
Where is a position vector, which acts along q1 from q2.
Clearly
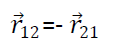
So from eqn 2 we get
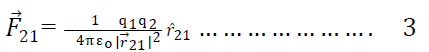
=
Compairing eqn 1 & 3 we get =
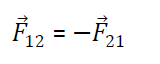
Hence Coulomb’s Law in vector from obey Newton’s third Law of motion also from diagram
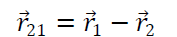 (from law of vector addition)
(from law of vector addition)
&
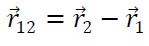 (from law of vector addition)
(from law of vector addition)
So eqn 1 & 2 becomes

Or
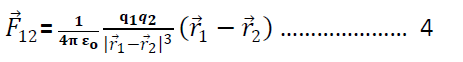
& similarly

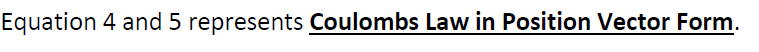
- Q .8: Four point charges qA = 2 µC, qB = −5 µC, qC = 2 µC, and qD = −5 µC are located at the corners of a square ABCD of side 10 cm. What is the force on a charge of 1 µC placed at the centre of the square?
Ans: 0
- Q.9. (a) Two small insulated charged copper spheres A and B have their centers separated by a distance of 50 cm. What is the mutual force of electrostatic repulsion if the charge on each is 6.5 × 10−7 C? (b) What is the force of repulsion if each sphere is charged double the above amount, and the distance between them is halved?
Ans: (a) force between the two spheres is 1.52 × 10−2 N. (b) 0.243 N.
13. Dielectric constant or relative permittivity:-
Permittivity is a property of the medium which determines the electric force between the charges situated in that medium.
Relative permitivity:-
As we know coulomb’s force between the two charge placed in vacuum is

Where o is the permittivity in free space
Again force between the same charges, when placed in a medium is
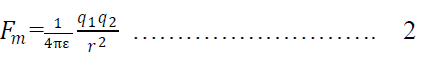
Here is the permitivity in the medium
Dividing eqn 1 & 2 we get

Where is is called relative permittivity.
Hence relative permittivity may be defined as the ratio of force between two charges in vacuum to the force between the charges in medium. Or Relative permittivity may be defined as the ratio of permittivity in the medium to the permittivity in free space.
Hence coulombs law for material medium becomes
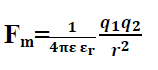
Q.10. What will be the Coulombs force if two charges are placed in water?
Clearly 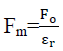
If charges are placed in water ( =80)then force between charges becomes

Here we can see that force reduces by 80 in water as compare to vacuum
14. Force Between Multiple charge: The superposition Principle:-
The principle of superposition states that if there are a number of charges exerting force on a single charge, then total force on single charge will be equal to sum of all the forces exerted by individual charge on the charge.
Suppose there are n charges q1, q2 , q3…qn exerting force on a charge q0.
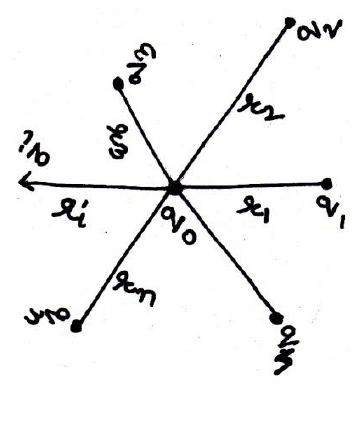
Then from coulomb’s law force on q0 due to q1
Is 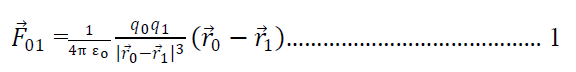
Again force on q0 due to q2 is 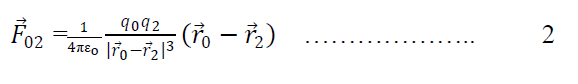
Similarly
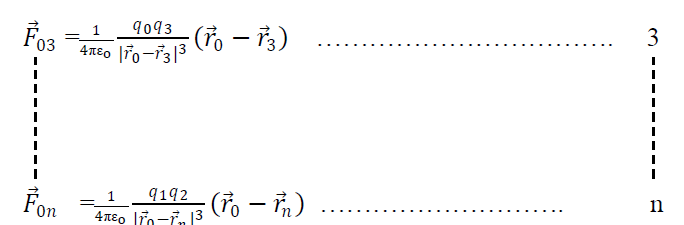
Adding all the eqn s we get
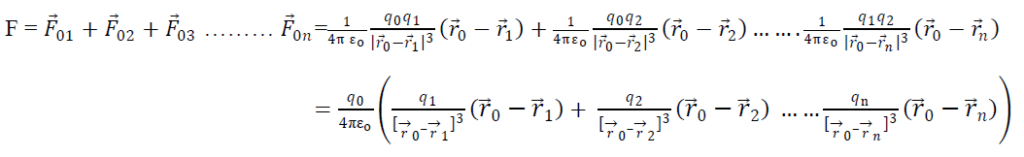
So total force on a charge

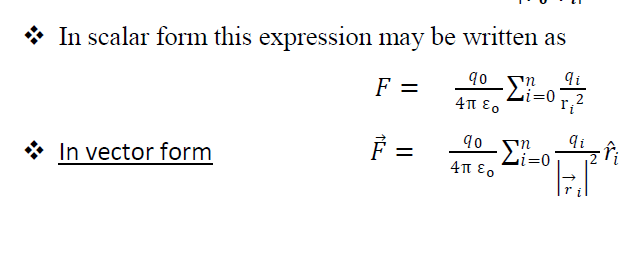
15. Force on a point charge due to continuous charge distribution:-
A continuous charge distribution is a system of charge lying at infinitelly small distances from each other.
There are three types of continuous charge distribution.
(a) Linear or line charge distribution:-
Ifcharges are arranged in such a way that they seem to be like a line then the distribution of charges is called line charge distribution.

The line charge density may be defined as the charge per unit length.
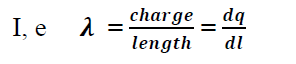
(b) Surface or area charge distribution:-
If charge are arranged in such a way that they seems to be a surface (plane)then the distribution of charge are called surface distribution of charge
The surface charge density may be defined as then charge per unit area of the
conductor.
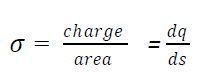
- Volume Charge distribution:-
If charges are arranged in such a way that they seems to be like a volume, then the distribution of the charges is called volume distribution of charge.
The volume charge density ρ may be defined as the charge per unit volume of the conductor.


16 force due to continuous distribution of charge:
(i) Forces at a point due to continuous line distribution of charges:-
Suppose a point P having r distance from a point O on continuous line distribution of charge. Than small force at charge placed at P due to small charge dq on small surface ds may be given by coulomb’s law,
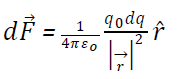
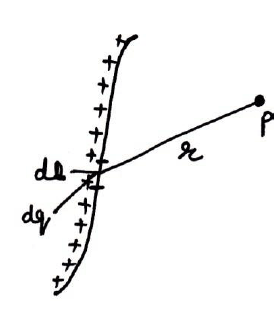

(ii) Force due to continuous surface distribution of charge:-
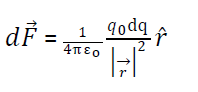
Where surface charge density


So the force at p due to complete surface is

- Force due to continuous volume distribution of charges.
Suppose a test charge is placed at point p having distance r from small volume dv having charge dq.
Then force on due to dq =
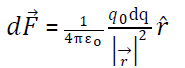
where volume charge density


Hence force at p due to complete surface is
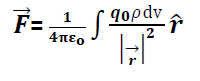
1(b) Electric Field